Glass is made from all-natural sustainable raw materials. It is the preferred packaging for consumers’ concerned about their health and the environment. Consumers prefer glass packaging for preserving a product’s taste or flavor and maintaining the integrity or healthiness of foods and beverages. Glass is the only widely-used packaging material considered “GRAS” or “generally recognized as safe” by the U.S. Food and Drug Administration. It’s also 100% recyclable and can be reused endlessly with no loss in quality or purity.
Glass Composition
Glass containers are commonly made with a combination of various oxides or oxygen-based compounds commonly referred to as soda-lime glass. Raw materials including sand, soda ash, limestone and cullet are combined to create glass containers that are durable, strong, impermeable, easily shaped, and inexpensive.
Some oxides will form glass without adding any other elements and are known as network formers. The most common of these is silica (SiO2).
The proportion of raw materials is based on availability - which GPI aims to increase - chemical and physical consistency, sizing, purity and cost. The goal is to use the most economical and high-quality raw materials available.
Sand
Sand is the most refractory of the major raw materials, or the hardest to melt; it is critical that it conform to fairly rigid sizing specifications.
The particle size distribution is typically between 40 (0.0165 inch or 0.425 mm opening) and 140 mesh size (0.0041 inch or 0.106 mm).
Sizing specifications for the other raw materials are dependent on the sand specifications.
Since larger particles of different sizes tend to segregate during material flow, the other materials must be sized to minimize the effects of this segregation.
Cullet
Cullet, or recycled glass, improves furnace efficiencies, including energy consumption. All cullet requires processing to remove non-glass contaminants and to create size uniformity:
Cullet is usually color separated, crushed to a maximum size of ¾ of an inch, and screened and vacuumed to remove contaminants.
Labels, aluminum caps, and non-magnetic metal are all considered contaminants.
Raw Materials & Uses
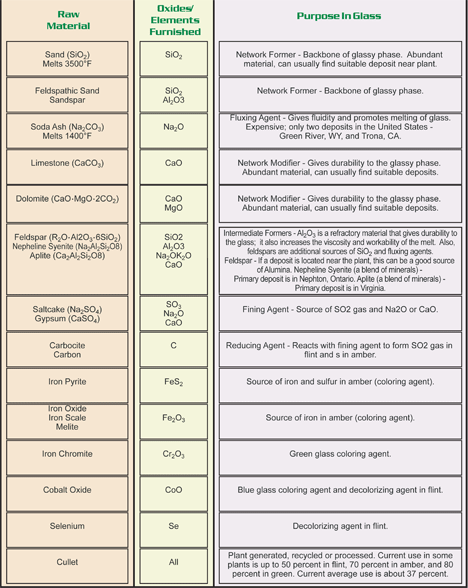
Principal Raw Materials Used In Glass
Minor ingredients such as fining agents, decolorizers, and colorizers are added to the typical container glass composition. The most common fining agents are sulfates in combination with carbon. Of the sulfates used, sodium sulfate, or salt cake, is the most common Sodium sulfate acts as a wetting agent to aid in melting the silica source and also as a fining agent.
Composition Control
Glass composition can be controlled by analyzing each new shipment of raw materials and its respective glass chemistry, requiring an analytical laboratory at each glass plant A far better method of composition control is to monitor certain easily measured physical properties of the glass that are influenced by the glass’ composition.
Two Methods for Composition Control:
Density
Density is defined as weight per unit volume. It is measured by comparison to a known standard using a sink-float technique, which can determine the density to the nearest 0.0002 grams per cubic centimeter.
Softening Point
The softening point for composition control is defined as the temperature at which a fiber of specified length and diameter will elongate at a rate of 1mm per minute, or the temperature at which the log of the viscosity equals 7.65. Using a fiber elongation technique, the softening point can readily be determined to the nearest 0.2 C.
Each oxide in the glass affects the Softening Point and Density differently:
- By measuring one of the physical properties daily, a change in glass composition can be detected by a change in that property. Since density is the easiest to measure and also the most accurate, this property is measured every day at all plants. Softening point is measured at certain plants as a second precautionary measure for ensuring composition stability.
- By using density as the measured property, glass composition can be controlled by Statistical Process Control techniques. By using such techniques it is possible to limit glass composition variations within detectable limits of wet chemical analytical techniques.

